2024-03-18

There is currently no information to display
2024-03-06
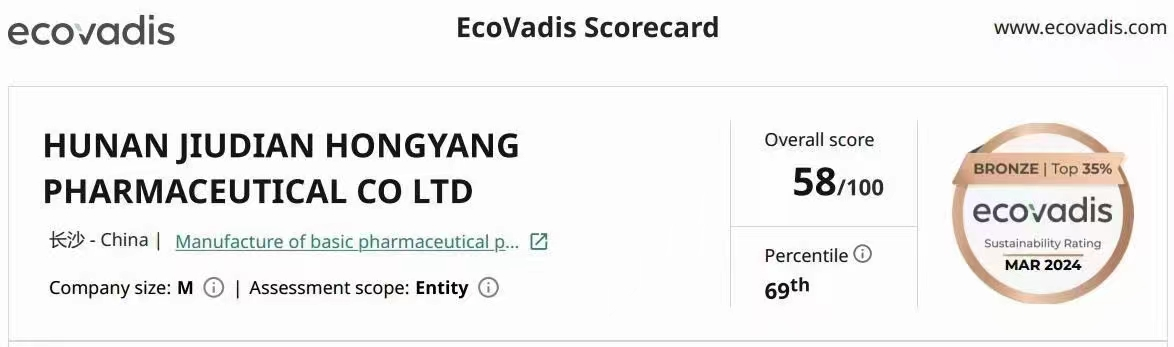
Hunan Jiudian Pharmaceutical have Ecovadis Bronze at the beginning of 2024
2024-02-29
Jiudian Pharmaceutical signed a patent and technology transfer agreement for LPS/CD 14 dual-target spot polypeptide new drug with Zonsen Peplib Biotech.
2024-02-03
Hunan Jiudian Pharmaceutical have Market Authorization of dapoxetine hydrochloride, celecoxib and Nicorandil API from NMPA
2024-02-01
Jiudian Pharmaceutical have drug license of Ebastine oral solution from NMPA
2024-01-24
Hunan Jiudian Pharmaceutical have Market Authorization of Methyl Salicylate and Sodium Sulfate APIs from NMPA
Previous page
1
2
Next page